Known as a reference in innovation, the country has one of the most challenging regulatory processes for foreign technologies in the world. The handheld fundus camera, developed and produced by Phelcom Technologies, is now commercialized in five countries.
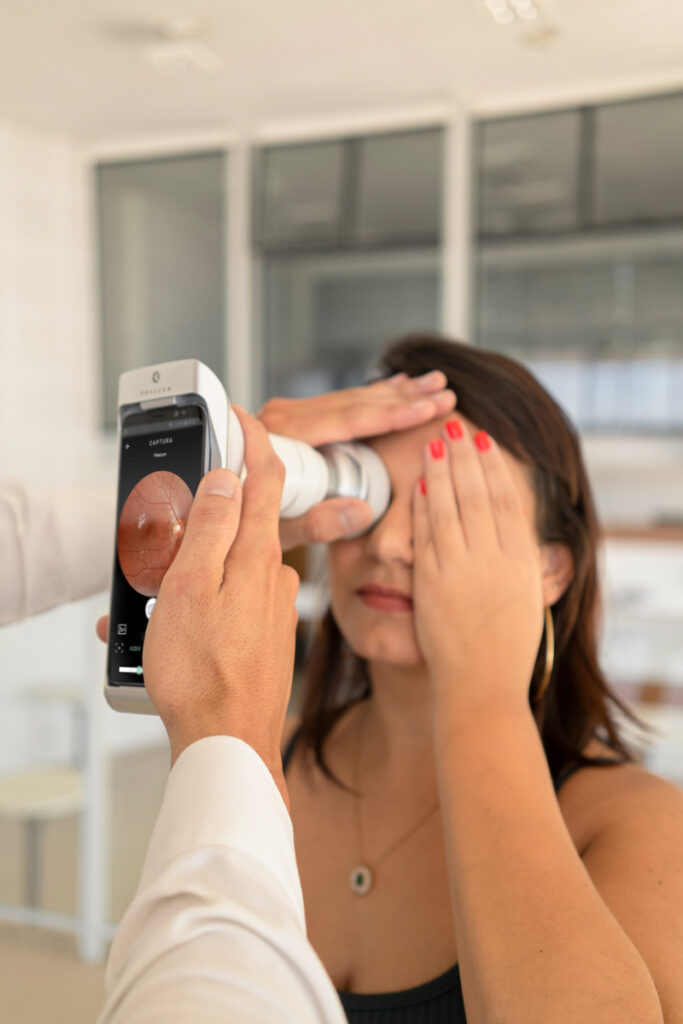
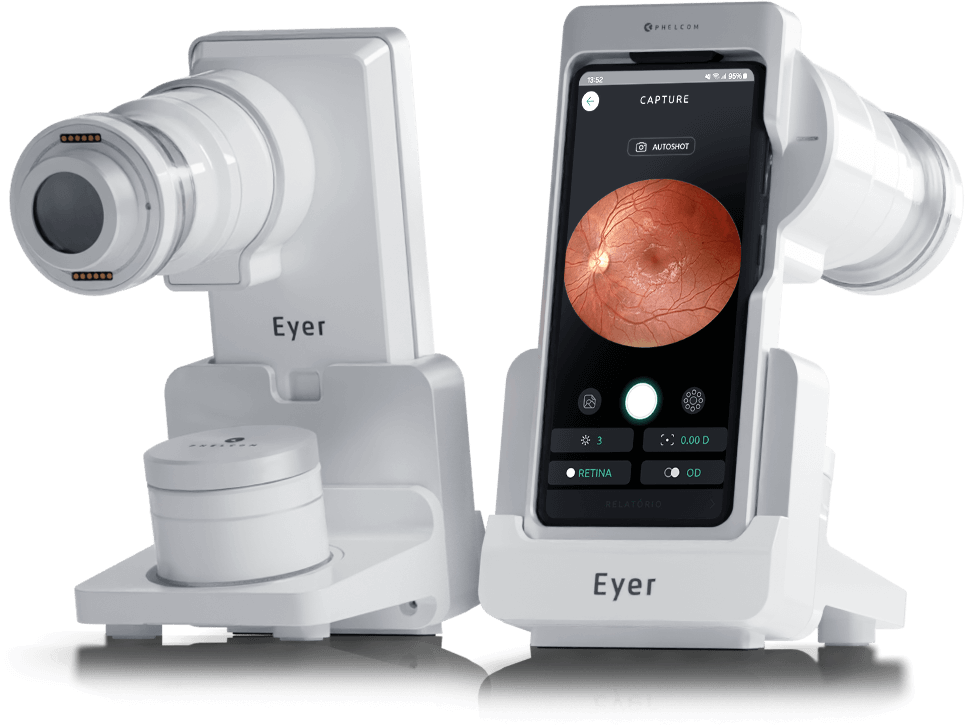
Phelcom Technologies has conquered one more international market. Now, Japan took its turn to receive Eyer, a non-mydriatic handheld fundus camera, coupled to a smartphone, that carries out high-quality retinal exams in a few minutes.
“We are very proud to have our equipment sold in a country known as a reference in technology and innovation”, states the company CEO, José Augusto Stuchi.
The Japanese regulatory process for entrance of foreign technologies is considered one of the most challenging in the world. This is due to the various players accessed in the procedure, such as DMAH – Designated Marketing Authorization Holder (legal representative), RCB (Regional Certifying Body), PMDA (regulatory agency); as well as specific certification workflows for each product class. The language barrier was another difficulty, since the whole reference documentation was in Japanese.
José Roberto Santiciolli Filho, Product Development coordinator at Phelcom Technologies, explains that the certification process started by the end of 2021, when the company was approved by the Ministry of Health, Labor and Welfare of Japan (MHLW) as a Foreign Manufacturer. All the manufacturing infrastructure and the Quality Management system were evaluated by then.
“After that, we developed a technical dossier of the product, according to PDMA standards, and submitted all the technical and quality documentation to a third-party unbiased evaluation to receive the certificate”, explains Santiciolli.
During this process, Phelcom counted on the support and partnership of Allm Inc., an investor based in Japan, for interlocution with local agents. Last November, Eyer received the medical device certification no. 304AIBZI00005000.
Eyer in the USA, Colombia and Chile
More than Brazil and Japan, Eyer is present in Chile, Colombia and the United States – where Phelcom also has an office in Boston. “This new unit confirms the internationalization movement of the company, started a year ago, and shall support the offer of equipment in the North-American market”, observes Stuchi.
The CEO highlights that physical presence in the United States confirms Phelcom’s availability to work for making eye exams simpler, connected and intelligent, without borders.
“In 2022, we took part, as expositors, of the main ophthalmology congresses of the United States, as the American Society of Retina Specialists Annual Meeting, in New York, and the American Academy of Ophthalmology, based at Chicago. American professionals have accepted Eyer very positively”, states Stuchi.
In March 2023, the company has also been at Vison Expo East, a commercial exposition in New York. This month, it is taking part of ARVO (April 23 to 27), in New Orleans, and ASCRS (May 5 to 8), in San Diego.
For Stuchi, being a Brazilian company which operates in important markets worldwide is a pride and great responsibility to Phelcom. “It is certain that internationalization widens our potential to act. However, it also increases our commitment to offering excellent quality products that really contribute to the professional practice”, he complements.
Phelcom Eyer
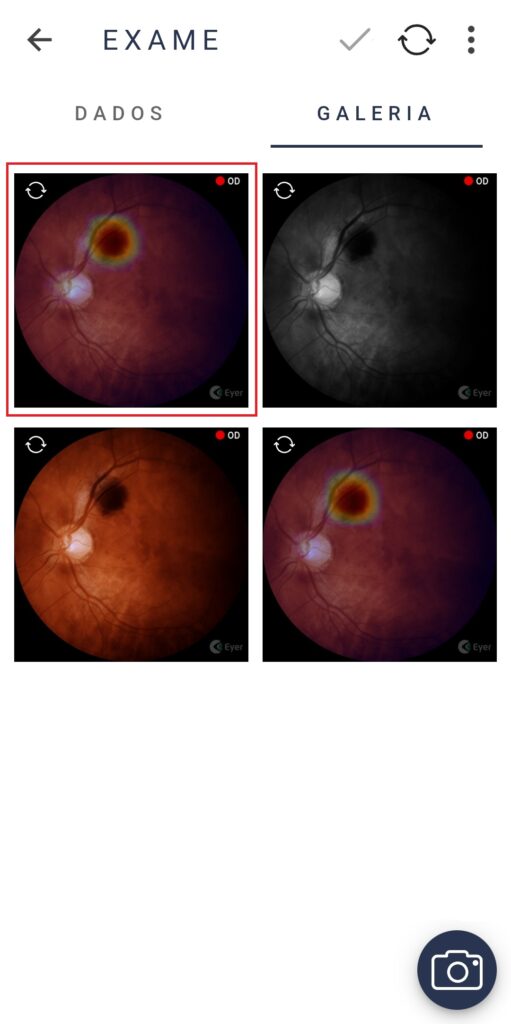
Phelcom Eyer is a handheld fundus camera that works coupled to a smartphone and carries out high-quality retina exams, in a few minutes, without need of pupil dilation.
The technology supports the diagnosis of over 50 diseases, such as glaucoma, cataract, diabetic retinopathy, DMRI, retinoblastoma, hypertensive retinopathy and ocular toxoplasmosis. Currently, more than 10 million exams have been made in Brazil, United States, Chile and Colombia.
The portability and the more accessible value of the technology democratize the access to retinal exams. Because it costs approximately six times less than a conventional tabletop fundus camera, which still needs to be integrated into a computer.
About Phelcom
Phelcom Technologies is a Brazilian medtech, headquartered in São Carlos, São Paulo. Its history started in 2016, when three young researchers – a physicist, an electric engineer and a computing engineer (PHysics, ELetronics, COMputing) – created a handheld fundus camera based in technology embedded in a smartphone.The interest of Diego Lencione, a company partner, on visual health gave birth to the first equipment project. His brother has a condition that severely compromised his retina and vision since childhood.
In 2018, Phelcom launched its first product in the market: Eyer handheld fundus camera. Nowadays, the technology has already reached more than 1.2 million people all over Brazil and other countries where it is present.
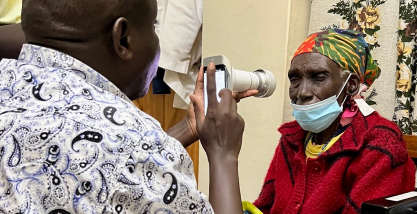
In four years, the company already took part of more than 100 social actions, such as the greatest joint effort of diabetes, in Itabuna (BA), and campaigns for Retina Global NGO in Sergipe and Kenya.
Recently, it was elected one of the 10 most innovative companies in Brazil by Forbes.