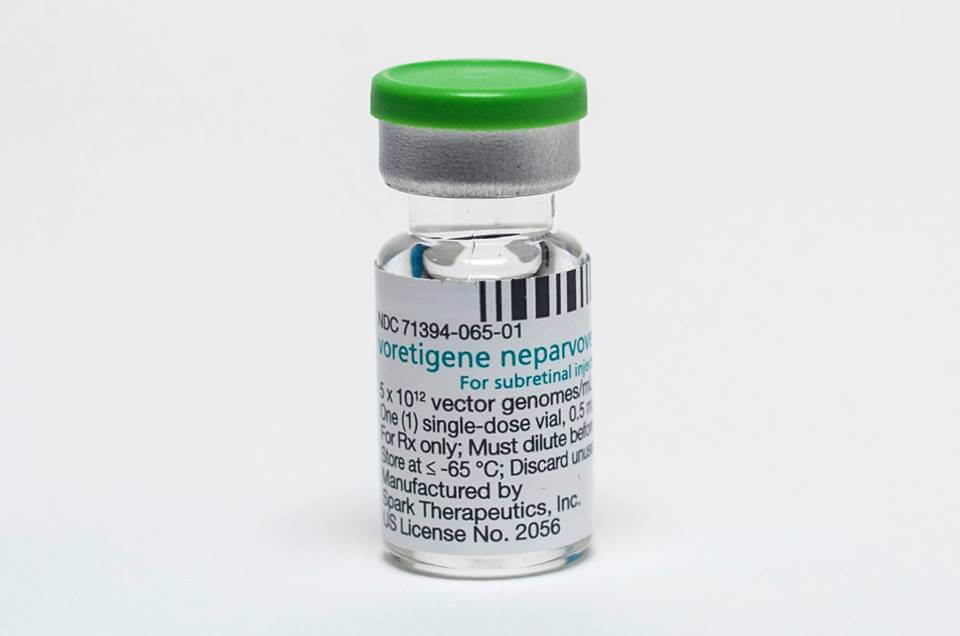
04Anvisa has approved the first product of gene therapy in Brazil, for treating Hereditary Retinal Dystrophy (HRD), as Leber congenital amaurosis and retinitis pigmentosa. There was no therapeutic alternative for the problem, so far.
Voretigene neparvopeque (trade name: Luxturna®) is a special kind of advanced therapy medicine. The enzyme it produces improves functioning of retina cells, decreasing the disease progress.
Learn more about this gene therapy, how it acts in the organism and its results to patients.
Gene therapy
In brief, gene therapy is based on inserting exogenous genetic material in a person’s cells for therapeutic purposes. Therefore, the transference aims to recover the function of a gene, provide a new gene function or intensify the functioning of active genes.
Objectively, gene therapy fixes defective genes by inserting healthy genes in patients with different diseases.
Hereditary retinal dystrophy
Hereditary Retinal Dystrophy (HRD) is a rare disease provoked by the mutation of human gene RPE65. Leber congenital amaurosis, retinitis pigmentosa, Usher syndrome and Stargardt disease are among the disorders it causes.
This gene mutation provokes gradual rupture of the cells that form the retina, located at the back of the eye. Therefore, it causes gradual loss of vision, culminating in blindness.
It usually occurs in childhood or teenage years. Though rare, it is the second cause of low vision in children up to 15 years old. Moreover, a considerable amount of its carriers evolves to complete blindness up to around 18 years old.
Some of the symptoms are difficulty or lack of adaptation to dark, loss of peripheral vision, abnormal color vision, photophobia and visual acuity loss, evolving to complete blindness.
How the medicine works
Elaborated through genetic engineering, the product consists of a virus with an inserted copy of the human gene RPE65, responsible for producing an enzyme necessary for the retina proper functioning.
Such enzyme allows a better performance of retina cells, thus decreasing the disease progress. During the study phase, it demonstrated gain of functional vision and autonomy in adults and children.
It is worth mentioning the virus used in manufacturing that medicine does not cause diseases in humans.
It is possible to treat children from 12 months old on and adults with vision loss due to hereditary retinal dystrophy. Novartis Biociências S.A. produces the medicine.
So far, there was no therapeutic alternative for the disturbance.
Another important remark: it is a hospital use product and must be used under expert medical supervision, via sub-retinal injection.
The physician also must take precautions, such as having the patient undergo mandatory tests able to prove his/her vision loss was due to mutations of RPE65 gene, over which the Luxturna’s active substance works.
Moreover, the doctor must use other lab and clinical evaluation criteria. For example, a certain amount of still functional retina cells is necessary for a successful treatment. If not, results may be low.
Conclusion
Gene therapy, as a whole, has enormous potential to treat diseases provoked by a single imperfect gene. That happens because, by fixing part of the defective genetic code, it solves or decreases the problem. It thus increases patient’s life quality.
Undoubtedly, this treatment brings up positive expectations towards rare diseases caused by genetic disorders.
Follow the main news on eye health on Phelcom blog.