The company’s production area of 160 m2 was approved by Anvisa.
In it, equipment class I and II may be produced by the company after registration.
The Phelcom plant is already structured and it will be possible to manufacture about 40 equipment per day with the installed infrastructure.
The next steps of the company will be the certification of the product in Anvisa, starting with the tests required by Inmetro.
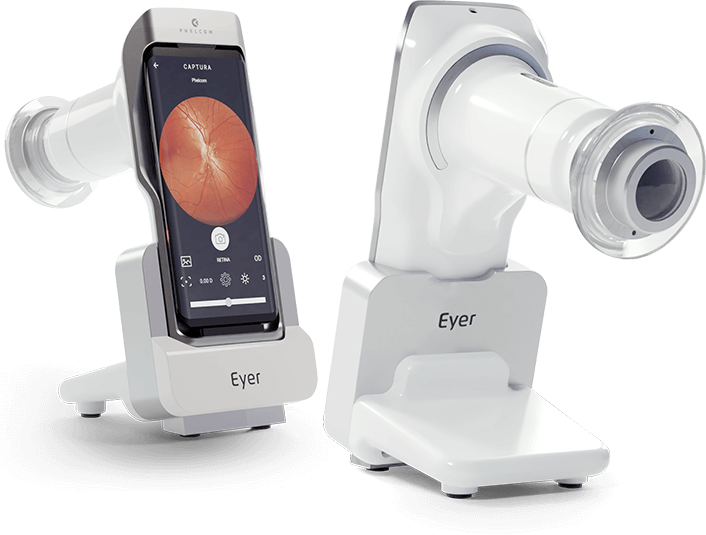
At the beginning of the year Phelcom is expected to launch its first product on the market, Eyer, a portable, connected and intelligent retinographer.