The company solidified its presence in the country by participating in significant events such as Vision Expo East, held from March 16 to 19 in New York, ARVO, from April 23 to 27 in New Orleans, and ASCRS, from May 5 to 8 in San Diego.
“These events bring forth many innovations, products, services, research, and advancements that not only keep us updated but also allow us to introduce Eyer to doctors, healthcare professionals, scientists, researchers, pharmaceutical companies, distributors, and potential partners in a relevant and swift manner,” emphasizes Phelcom’s International Manager, Mário Costa.

FDA Approves Eyer as 510(k)/FDA Cleared
Recently, the FDA (Food and Drug Administration) granted approval for Phelcom’s portable retinal camera, Eyer, as 510(k). Previously considered an exempt device, the technology was limited to patient photo documentation.
“Now, as we are FDA Cleared, Eyer can be used for diagnostic and telemedicine purposes. This new classification, the highest in our category, opens up more opportunities for our American clients to use our equipment both inside and outside the office,” Costa highlights.
Upcoming International Events
Phelcom will take part in several international trade shows and congresses in the second half of the year. Check out the schedule:
AACO ANNUAL CONFERENCE 2023
August 18-20, Texas (US)
https://www.aacoeyes.org/2023-annual-conference-austin-tx
VISION EXPO WEST 2023
September 27-30, Las Vegas (US)
https://west.visionexpo.com/
ACADEMY 2023
October 11-14, New Orleans (US)
https://aaopt.org/meetings/annual-meeting/
AAO 2023
November 3-6, San Francisco (US)
https://www.aao.org/annual-meeting

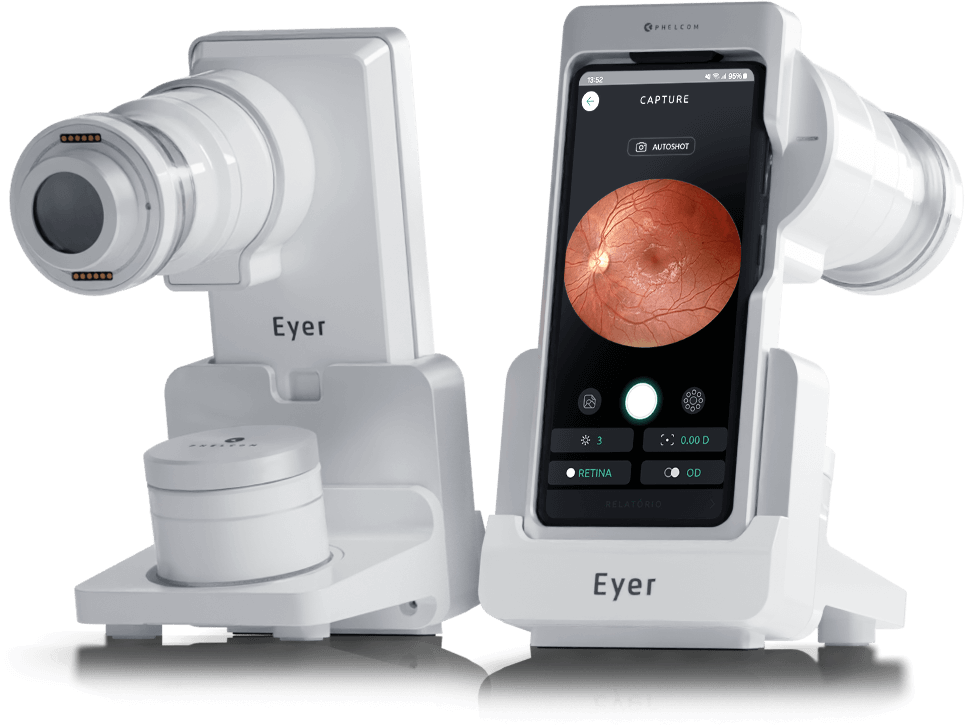
Phelcom Eyer
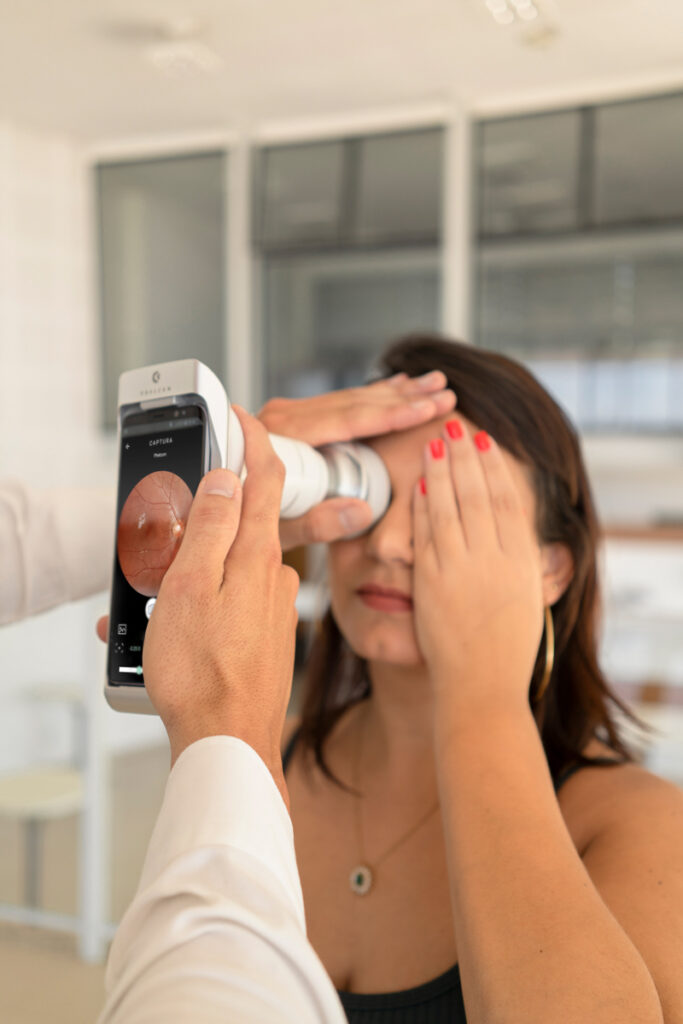
Phelcom Eyer is a portable retinal camera that attaches to a smartphone and conducts high-quality retinal exams within minutes without the need for pupil dilation.
This technology aids in diagnosing over 50 diseases, including glaucoma, cataracts, diabetic retinopathy, AMD, retinoblastoma, hypertensive retinopathy, and ocular toxoplasmosis. It is available in Brazil, the United States, Japan, Chile, Colombia, and will soon become available in other countries.
Portability, connectivity, and integration with intelligent functions like EyerMaps, combined with the technology’s accessible price point, contribute to increased access to retinal exams.
About Phelcom
Phelcom Technologies is a Brazilian medtech company headquartered in São Carlos, São Paulo. The company’s story began in 2016 when three young researchers – a physicist, an electronic engineer, and a computer engineer (PHysics, ELectronics, COMputing) – created a portable retinal camera integrated with a smartphone.
The idea for the first prototype arose from the interest of partner Diego Lencione in visual health, driven by his brother’s condition, which severely affected his retina and vision since childhood.
In 2019, Phelcom introduced its first product to the Brazilian market: the portable retinal camera Eyer. More than 2 million people in Brazil and around the world have been examined using Eyer so far.
In four years, the company has participated in more than 100 social initiatives and was recently named among the 10 most innovative companies in Brazil by Forbes.