Brazil, United States, Chile, Colombia and, now, Japan. Eyer portable fundus camera, developed and produced by Phelcom, has just been approved in the country.
The Japanese regulatory process for entry of foreign technologies is considered one of the most challenging in the world. This is due to the various players accessed during the procedure, such as the DMAH – Designated Marketing Authorization Holder (legal representative), RCB (third-party certification organization), Foreigner Manufacturer and PMDA (regulatory agency), besides the specific certification flows for each product class. The language barrier was another difficulty, since all the reference documentation was in Japanese.
José Roberto Santiciolli Filho, coordinator of Product Development in Phelcom Technologies, explains that the certification process started in the end of 2021, when the Japanese Ministry of Health, Labour and Welfare approved the company as a Foreign Manufacturer. At this stage, all the manufacturing infrastructure and Quality Management system were evaluated.
“After that, we developed the technical dossier of the product, according to PMDA standards and submitted all the technical and quality documentation to third-party impartial evaluation, for then receiving the certificate”, explains Santiciolli.
During the certification process, Phelcom counted on the support of its investor, Allm Inc., headquartered in Japan, for interlocution with local agents. In November this year, Eyer received the medical device certification with the number 304AIBZI00005000.
Phelcom Eyer
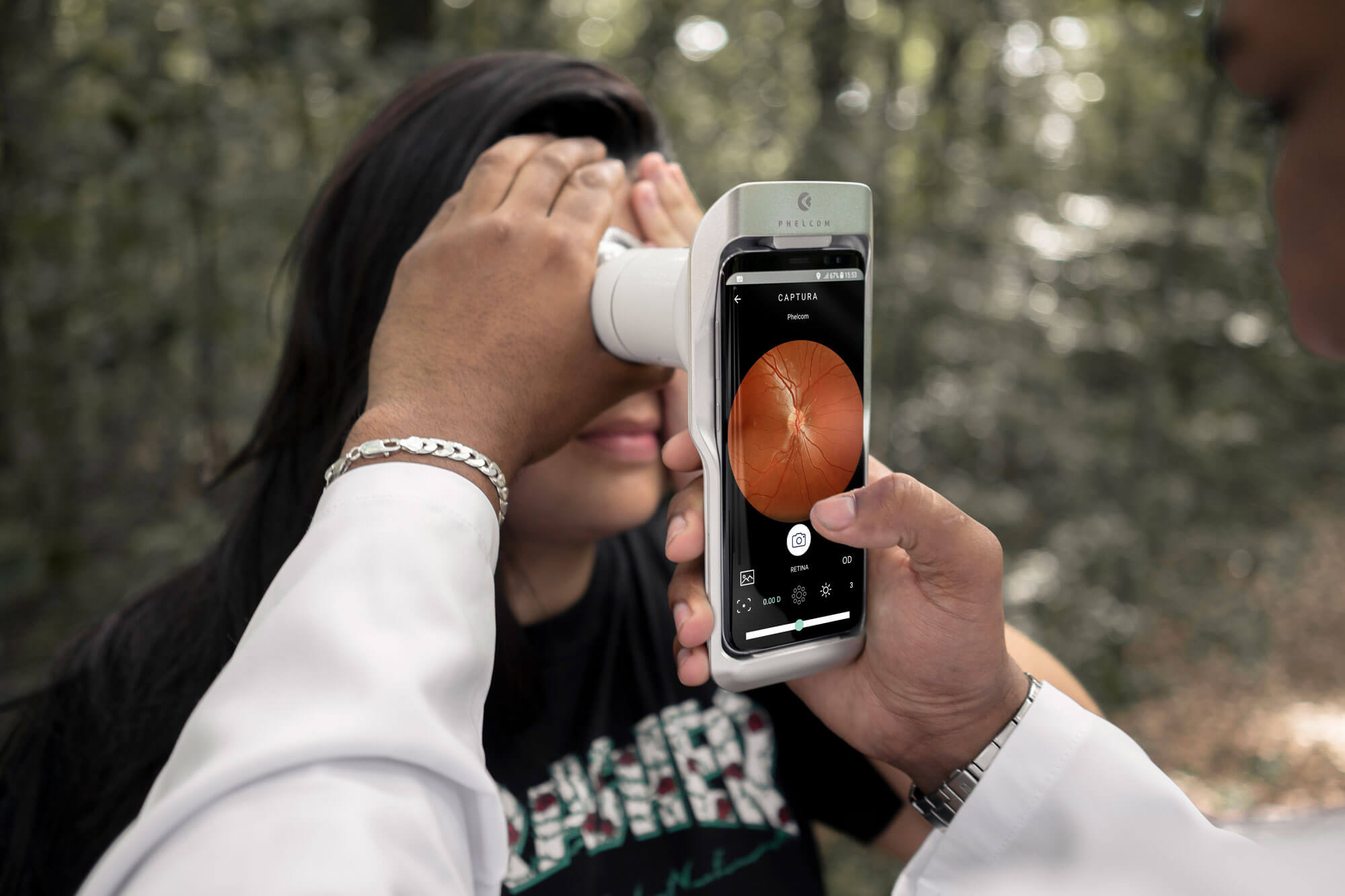
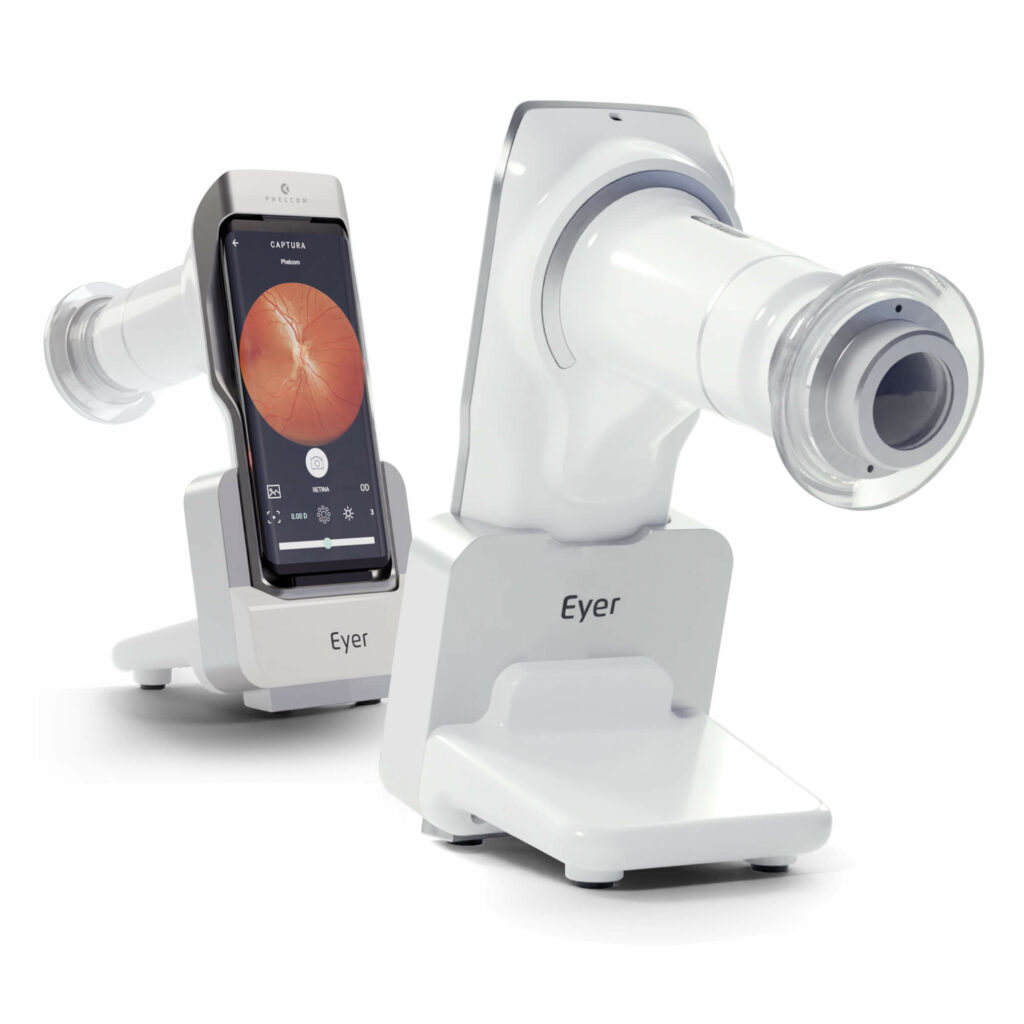
Phelcom Eyer is a handheld fundus camera that works coupled to a smartphone. It carries out high-quality fundus exams in a few minutes, without the need of pupil dilation.
Integrated to an online platform, EyerCloud, data are automatically sent and can be analyzed by a specialist from anywhere in the world. That is, it makes remote diagnosis possible.
More than that, the embedded artificial intelligence provides intelligent functions to help medical diagnosis and capture of retinal exams. On the other hand, the portable technology of accessible value democratizes the access to retinal exams. The device is around six times cheaper than a conventional tabletop fundus camera, which still demands integration to a computer.
Learn about the advantages:
CONNECTIVITY
The device is naturally connected, since it is attached to the smartphone. Therefore, it eases cloud sharing and access of exam data in the Eyer Cloud system.
NON-MYDRIATIC
Eyer allows to carry out retinal exams anywhere, without needing eye drops for pupil dilation. Thus, the patient feels more comfortable and the exam is faster.
LOW COST
Portability and reduced size grant Eyer a lower cost in relation to traditional fundus cameras. Even with the cutting-edge technology applied in the device production.
Currently, more than 1.2 million people have been examined and around 10 million exams were carried out in Brazil, United States, Chile and Colombia.
Eyer is also in the certification process in Mexico. Furthermore, a new Phelcom product is being approved in Brazil and in the United States.
“The favorable result of the process in Japan demonstrates we are on the right track to democratize access to ophthalmology. We are very happy to make available to one more country an innovative solution that will make a difference in the struggle against blindness in the world”, highlights Santiciolli.

 Português
Português  Español
Español  English
English